
In the denitrification part of the nitrogen cycle, nitrates react completely to form molecular nitrogen, where nitrites, NO and N2O are stepwise reaction intermediates. For every step, there is a different reductase. In facultative anaerobic bacteria, the nitrate reductase is a transmembrane protein that associates with a nitrate/nitrite antiporter. The nitrite reaction product is delivered to the periplasmic space, where the enzymes for the next two reaction steps are localized (nitrite and NO that escape to the cytoplasm are toxic).
Currently, there are two distinct classes of nitrite reductases known: class I enzymes contain copper as a cofactor, while class II enzymes use a heme group. In the conversion of ammonia to nitrites in the nitrification cycle, there are even hexaheme-containing nitrite reductases.
The molecular structures of class I and class II enzymes have been solved.
Class II enzymes catalyze the one electron reduction of nitrite to NO and water:
NO2- + 2 H+ + e- -> NO + H2O
as well as the four electron reduction of oxygen to water:
O2 + 4 H+ + 4 e- -> 2 H2O
Because of its heme content, the class II enzyme is also designated cytochrome cd1. Nitrite reduction is approximately ten times more efficient than oxygen reduction in denitrifying bacteria, and it is therefore regarded as the physiologically relevant reaction.
The copper-containing class I enzymes are inactivated by oxygen in a reaction releasing hydrogen peroxide.
| NIR class I | NIR class II | |
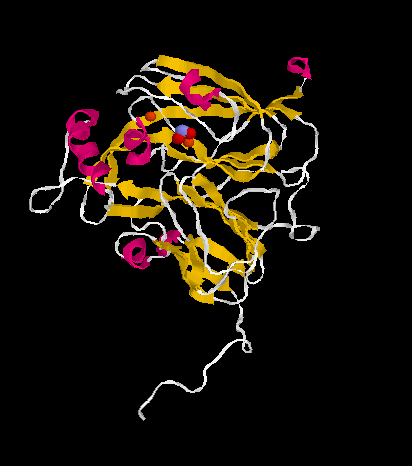 |
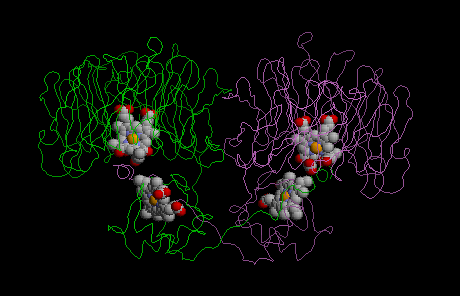 |
|
| structural details | structural details |
NO is a product of nitrite reduction, and is reduced by nitrogen monoxide reductases to N2O:
2 NO + NADH + 2 H+ -> N2O + NAD+ + H2O
No further proteins take part in this reaction; the electrons are directly exchanged between NAD and the prosthetic heme group. The enzyme from denitrifying mushroom Fusarium oxysporum has been crystallized both in the substrate-bound and apo forms.
| NOR F. oxysporum |
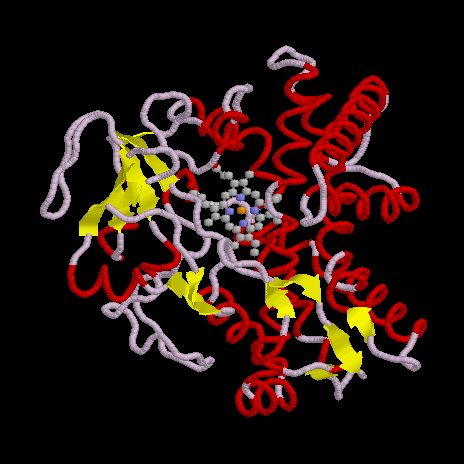 |
| structural details |
Literature: WG Zumft, Cell Biology and Molecular Basis of Denitrification. Microbiology and Molecular Biology Reviews 61 (1997) 533-616